Abstract
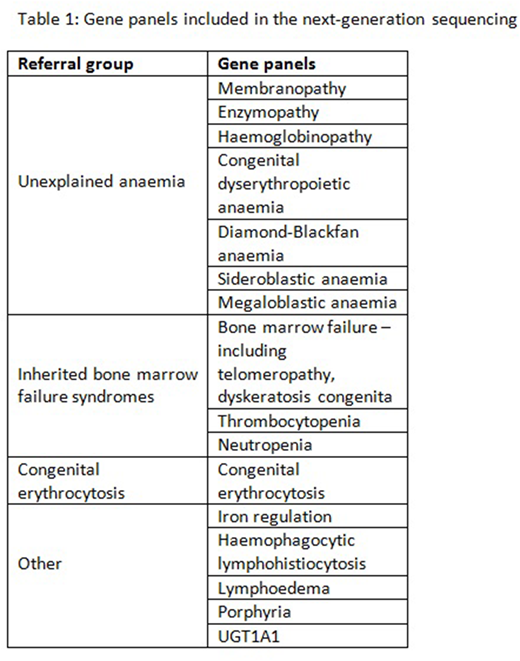
The genetic diagnosis of inherited anaemias is an important aspect of the diagnostic pathway for patients with haematological disorders, allowing discrimination between conditions of overlapping phenotypes therefore enabling more effective clinical treatment. Next Generation sequencing platforms are now in widespread use in diagnostic settings and are facilitating more rapid, accurate and cost-effective molecular diagnosis. The Red Cell Gene Panel developed by the Viapath Molecular Pathology laboratory based at King's College Hospital, London has harnessed this technology with the aim of identifying genetic diagnoses of rare inherited causes of anaemia. Although originally setup to diagnose inherited red cell disorders, clinical demand has led to the inclusion of inherited bone marrow failure syndromes and other related conditions such that the panel now consists of 194 genes, divided into 16 subpanels (see table 1).
Here we present the data from the first 1000 diagnostic cases reported under the following referral groups: 462 cases of unexplained anaemia (including haemolytic anaemia, sideroblastic anaemia, congenital dyserythropoietic anaemia, Diamond-Blackfan Anaemia), 232 cases of inherited bone marrow failure syndromes (including thrombocytopenia and neutropenia), 163 cases of congenital erythrocytosis and 143 other cases (including but not limited to iron regulation, haemophagocytic lymphohistiocytosis (HLH) and Criggler-Najjar ). Of these 1000 cases, we have achieved an overall diagnostic yield of approximately 25%. A diagnosed case is defined here as one in which a clear pathogenic or likely pathogenic variant that explains the phenotype has been detected. The unexplained anaemia cases have achieved the highest percentage of cases diagnosed with 47% diagnostic yield and data will be presented outlining the gene-by-gene breakdown of diagnoses made.
Our bespoke bioinformatics pipeline has also allowed the detection of novel disease-causing structural variants in 20 cases, contributing 2% of our diagnostic yield. These are detected using three different methods; read-depth analysis, split-read mapping and discordant insert-size analysis. All reported structural variants have been confirmed with a second method, either breakpoint mapping or dosage-sensitive PCR.
A significant proportion of cases (28%) have been reported with variants of uncertain clinical significance, highlighting the need for family studies and functional characterisation to be able to accurately ascertain the significance of these variants. Future developments of the service include functional characterisation of membrane disorders using next generation ektacytometry and preliminary data from this work will be presented here.
Kulasekararaj:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support . Pagliuca:Gentium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.